Vaccine Adjuvants

S-540956
S-540956, a novel Toll-like receptor 9 (TLR9)-agonistic adjuvant consisting of B-type CpG ODN2006 (also known as CpG7909), annealed to its complementary sequence oligodeoxynucleotide (ODN) conjugated to a lipid. CpG oligodeoxynucleotides are synthetic single stranded DNA fragments containing unmethylated CpG motifs that mimic bacterial DNA. CpG ODNs can strongly activate plasmacytoid dendritic cells (pDCs) and B cells via the TLR9 signaling pathway and promote the establishment of adaptive immunity. Albumin is intrinsically transferred to lymph nodes (LNs), and thus, molecules that bind albumin are effectively delivered to LNs. S-540956 contains lipid and binds to albumin, and this enable S-540956 to accumulate efficiently in LNs by hitchhiking albumin. LNs are crucial for orchestrating innate and adaptive immune responses. Our researches have demonstrated robust adjuvant effect by mixing with antigens such as cancer peptides and recombinant viral antigens without enhancing systemic inflammation.
■ Product profiles of S-540956
Mechanism of Action |
Activation of TLR9 signaling pathway |
|---|---|
Application |
• Vaccine adjuvant for infectious disease • Cancer immunotherapy/vaccine |
Administration Route |
Intramuscularly or subcutaneously |
Stage |
IND application for Phase 1 trial |
Schematic structure of S-540956

■ Characteristics of S-540956
From the characteristics of S-540956, it is expected to serve as a vaccine adjuvant for infectious disease (especially, chronic infection such HBV, HPV, HIV, HSV-2, Mycobacteria, etc) and cancer immunotherapy/vaccine.
■ S-540956 efficiently accumulates at LNs as designed
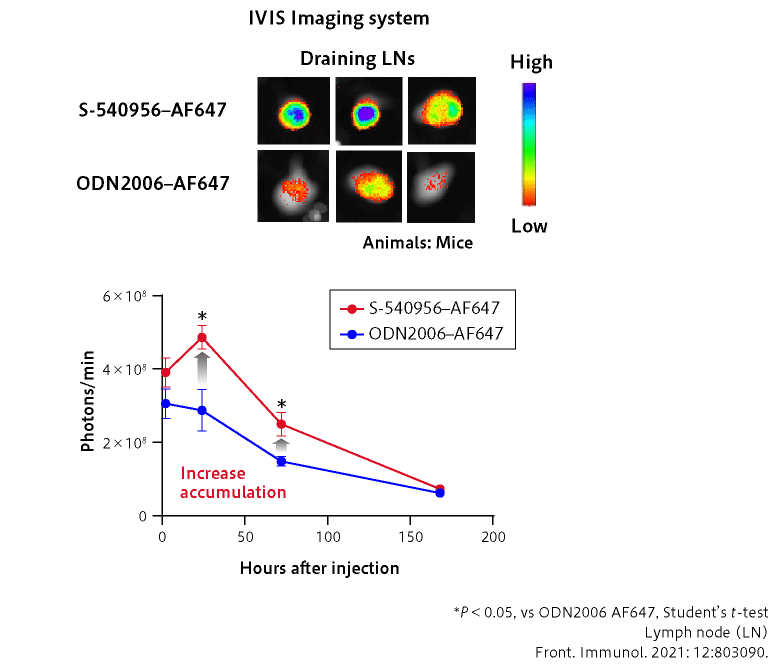
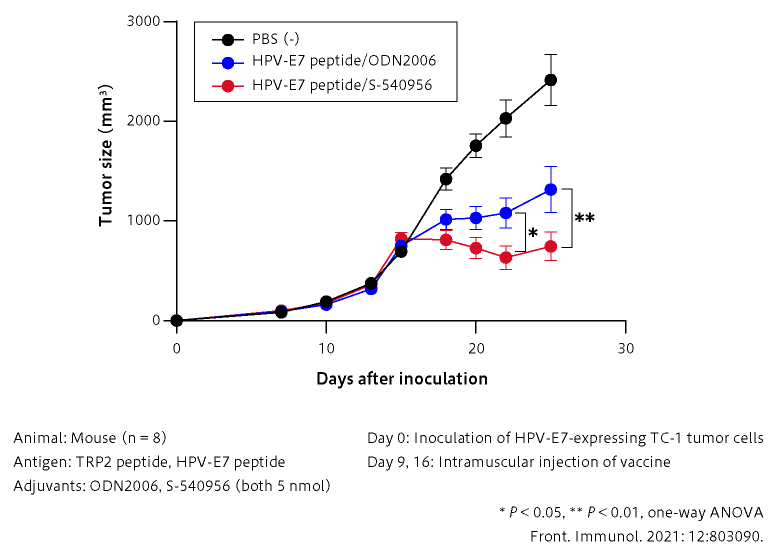
■ Therapeutic effect of the cancer peptide vaccine is significantly enhanced by S-540956
A-910823
A-910823 is a squalene-based, oil-in water emulsion adjuvant developed by Shionogi & Co., Ltd. In the studies, the combination of antigen with the adjuvant is tolerated and the induction of neutralizing antibodies and induction of antigen-specific T cells responses toward Th1 are confirmed. Thus, A-910823 can potentially accelerate the development of novel effective vaccines.
If you have any questions about our adjuvants or requests for more information, please contact us using the following form.
