Risk Management
Promoting risk management system
SHIONOGI takes appropriate management actions to create business opportunities and avoid or mitigate risks. At the same time, it employs the Enterprise Risk Management (ERM) system, which oversees the entire Group’s business risks, including risks of crises such as pandemics, natural disasters, terrorism, and cyberattacks, as an important mechanism for management strategy and management foundations.
In operating and managing the ERM, we classify the significant risks assessed as having the potential to have a material impact on our business performance and management into two categories: “business strategy-related risks,” which are inherent in strategic decision making and may inhibit the implementation of strategies, and “business execution-related risks,” which may affect the implementation of business operations that support management objectives. By clarifying responsibilities and ensuring transparency in the status of countermeasures, we conduct comprehensive risk management. These risks are discussed and approved at the Corporate Executive Meeting and meetings of the Board of Directors.
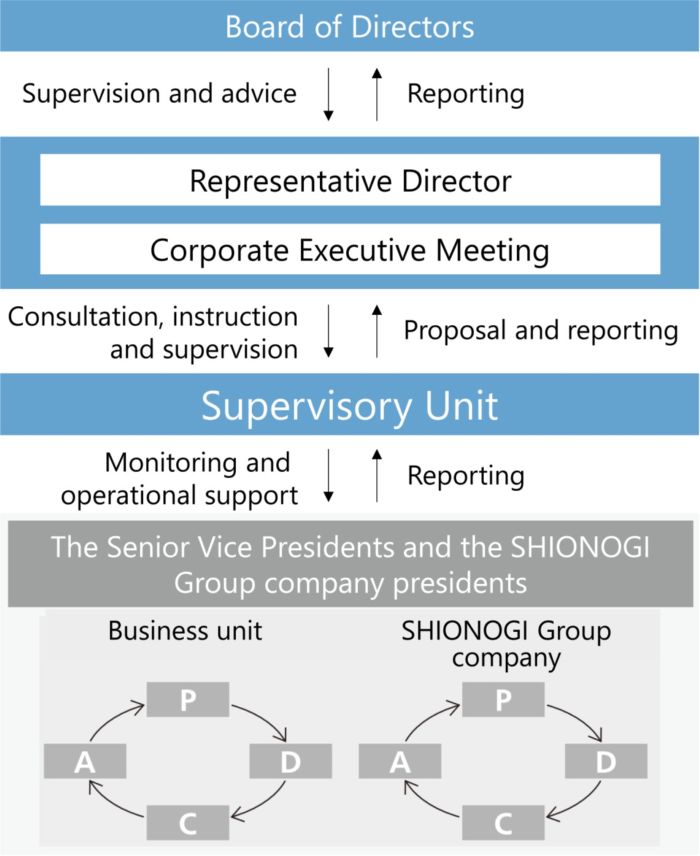
Business strategy-related risks and business execution-related risks are discussed every quarter at the Corporate Executive Meeting, where risk lists are updated, risks that need to be addressed are identified, and responsible units are appointed. Each responsible unit works with other units and relevant organizations to formulate and implement plans to take advantage of or reduce uncertainties, and the progress is monitored at the Corporate Executive Meeting.
The unit is also responsible for appointing risk owners for risks under control of the unit to monitor them, and taking into consideration the impact and likelihood of occurrence of the risks, escalating them to the Corporate Executive Meeting as business strategy-related risks or business execution-related risks, as necessary. By adopting a risk management system centered around these responsible units, we are able to swiftly and flexibly identify issues and plan and promote countermeasures even during the fiscal year.
Regarding crisis management, based on the crisis management rules, etc. and under a comprehensive management system, including a business continuity plan, we promote management that focuses on respect for human life, consideration for local communities and contributions, and control of corporate value damage. In the event of a crisis, we will deal with it and overcome it promptly.
In addition, in order to enhance the effectiveness of risk management, we also focus on the development and operation of a system to ensure proper business operations, based on the Basic Policy for Construction and Operation of the Internal Control System.
These activities are regularly reported to the Corporate Executive Meeting and the Board of Directors to receive advice for improvement from Directors. We have thus established a system to supervise risk management activities.
Fostering corporate risk culture
Fiscal Year |
Initiatives | contents |
|---|---|---|
| FY2020 | All Employee Education | Understanding business risks and making decisions based on business risk levels |
| FY2021 | All Manager Education | Making decisions based on business risk levels |
| FY2022 | All Employee Education | "SHIONOGI Group Risk Management Policy" Foundation of ERM (Enterprise Risk Management) knowledge Education |
| All Organizational Leaders Education (Responsible for executing risk management) | ERM structure and respective roles Basics of risk management Education |
|
| ERM leaders in each department and group company Education | Introduction of company-wide risk management system Officer Education (Newly Appointed) | |
| Officers Education (newly appointed) | SHIONOGI's ERM structure and policies SHIONOGI Group Risks |
|
| Risk Assessment in Supervisory Unit Meeting (by Business Unit) | Risk Assessment Implementation (Risk Identification, Evaluation, and Analysis). Discussion on the validity of risk assessment and progress monitoring of risk response |
|
| FY2023 | Risk Identification in Corporate Executive Meeting | Identification and Progress Monitoring of "Risks to be Monitored in Management Meetings" External Disclosure divided into two categories based on Management Meeting Discussions ("Business strategy–related risks" and "Business execution–related risks") |
Main Business risks
At SHIONOGI, we categorize risks that could have a material impact on performance and business as either “business strategy-related risks,” risks inherent in strategic decision-making, or that could hinder the implementation of the strategy, or “business execution-related risks,” risks that impact business execution that support business targets.
1. Summary of business strategy–related risks
1.Global growth centered on the infectious disease area
≪ Risk Summary ≫
In the infectious disease area, revenue is easily influenced by trends, and market predictability is lower than in other disease areas. Even successful drug development sometimes does not lead to recovery of investment. In addition, novel antibiotic drugs are mainly used for the purpose of reducing the incidence of resistant bacteria and are only used when treatment options are limited, which makes market prediction difficult for antibiotic drugs. On the other hand, given the recent rising public concerns about infectious diseases, SHIONOGI sees these as an opportunity and has set “Protect people worldwide from the threat of infectious diseases” as one of its material issues. SHIONOGI works to establish an optimal revenue model by combining “antimicrobial resistance (AMR) drugs,” “anti-HIV drugs,” “vaccines,” and “acute respiratory infection drugs,” thereby making its overall disease business sustainable. For acute respiratory infections, in particular, we aim to advance from a stage of stability to a stage of growth by globally deploying treatment and preventive drugs for influenza, COVID-19, and Respiratory Syncytial Virus (RSV) infections. SHIONOGI has so far launched many products overseas mainly through partnerships with partner companies, in which it has received a part of the product sales as royalties. While maintaining good relationships with these partners, particularly in the HIV business, SHIONOGI should also enhance its global business development so that it can take the initiative in development, regulatory affairs, marketing and sales, and other business activities for SHIONOGI products in Europe, the U.S., and Asia, as well as activities to improve access to medical services in lower-middle income countries. However, if the initial development plan or sales strategy is delayed or fails, or if the expected discovery of treatment drugs or vaccines or sales revenue cannot be realized, it may significantly affect our performance.
≪ Major responses and initiatives ≫
- Sales and promotion of proper use of COVID-19 and influenza drugs
- Expansion of sales of cefiderocol, an antimicrobial resistance (AMR) drug
- Development of COVID-19 and influenza preventive vaccines
- Research and development on long-acting treatment and preventive drugs to improve the QOL of people living with HIV
- Research and development on new treatment of infectious diseases with high unmet needs (tuberculosis, malaria, non-tuberculous mycobacterial diseases, etc.)
- Creation of products and services that realize total care for diseases, including pre-symptomatic care, prevention, testing, diagnosis, and convalescence, in addition to treatment
- Implementation of global development and application for approval for products launched overseas, and improvement of overseas production, distribution, and sales systems
- Negotiations with governments and regulatory authorities of various countries on stockpiling and expansion of the subscription-type reimbursement model
2.Expanding pipelines
≪ Risk Summary ≫
Aiming to realize a society in which everyone can live life to the fullest and on their own terms, SHIONOGI engages in research and development on drugs and other healthcare solutions that will help solve problems of people. Research and development of pharmaceuticals require a long period of time and a large amount of investment. There is also a possibility that the expected effects cannot be obtained in clinical trials, resulting in failure to obtain approval. In this environment with high uncertainty, in order to raise the success rate of drug discovery and build attractive pipelines that satisfy medical needs, in addition to the research and development technologies SHIONOGI has cultivated for infectious diseases and small molecule drugs, it is crucial to acquire new modalities, utilize external networks, obtain growth drivers from outside through active investment, and develop human resources capable of handling these operations. We are also working to shift from a conventional business model relying heavily on revenue from pharmaceutical patents and start providing vaccines and new healthcare services, as we see this as an opportunity to solve diverse problems of patients and society that cannot be solved by pharmaceuticals alone. We believe that by balancing the prescription drug business and other businesses, fluctuations in revenue due to patent expiration can be mitigated.
≪ Major responses and initiatives ≫
- Venturing into new modalities and technologies
- Promotion of co-creation with external parties
- Active investment in growth drivers, such as in-licensing
- Human resource development to secure cutting-edge research and development capabilities
- Maintaining a high original pipeline ratio
- Development of innovative treatment options beyond conventional treatment drugs, such as digital apps
- Development of services necessary to create an environment in which all people can play active roles
3.Human capital management
≪ Risk Summary ≫
In order for SHIONOGI to transform its business model and achieve the growth targeted by the STS2030 Revision, each and every employee should constitute the “source of competitiveness” that leads the transformation, and SHIONOGI should be a group composed of such diverse human resources with strengths. To this end, we see human capital management toward achieving the Vision for 2030 as a business opportunity and have set it as a key theme in our strategy. By recruiting external human resources and promoting ability-based appointment, we will transform our personnel portfolio and realize a fusion of human resources with diverse values, thereby aiming to achieve our 2030 Vision. SHIONOGI has established “SHIONOGI Way: Be the best that you can be to take on new challenges by constantly improving and expanding your capabilities” as its vision for human resources, and it implements various measures to help employees to acquire both the abilities that all employees should have and the abilities that are required for individual roles. We will also enhance mid-career recruiting to acquire expertise that SHIONOGI lacks. In addition, by developing various systems and programs, we have been establishing an environment in which diverse people are able to enhance their engagement and play active roles. Furthermore, we are also working to ensure the health and safety of our employees, which are necessary in carrying out these initiatives. However, if any obstructive factor, such as failure to implement measures or acquire human resources or the occurrence of an incident that affects the health and safety of employees, impairs the value of SHIONOGI’s human capital and delays SHIONOGI’s reform, it may have a significant impact on our performance.
≪ Major responses and initiatives≫
- Enhancing mid-career recruitment
- Revision of personnel system
- Workstyle reform to enable various characteristics to play active roles
- Holding events to encourage and praise those who take on challenges
- Enhancing operation of the EHS management system at each operating site
- Promotion of setting OELs for worker exposure to APIs
- Setting and implementation of handling standards for worker exposure
4.Realizing reform by DX
≪ Risk Summary ≫
Seeing recent technological innovations and the dynamic changes in the environment surrounding them as an opportunity, SHIONOGI declares in the STS2030 Revision its commitment to digital transformation through various activities as a big theme to accelerate decision making and realize new value creation based on data. As conventional business models are required to be transformed, it is essential to improve productivity using AI and IT. If the efforts to achieve this become stalled, it may have a significant impact not only on the performance of SHIONOGI but also on the improvement of its corporate value.
≪ Major responses and initiatives ≫
- Building global IT infrastructure
- Business model/operation reform through practicing AI drug discovery, market inventory prediction using AI, etc.
- Developing a medical device program (SaMD) for diagnosis and treatment of diseases, and disease detection algorithms
- Establishing a data utilization base that will improve work efficiency and realize new value creation
- Implementing measures to develop digital core human resources
2. Business execution–related risks
1.Quality
≪ Risk Summary ≫
SHIONOGI manufactures products and outsources manufacturing under strict quality control in compliance with Good Manufacturing Practice (GMP) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines and other pharmaceutical-related laws and regulations. It has also been inspected by competent agencies, such as the Ministry of Health, Labour and Welfare in Japan, the Food and Drug Administration (FDA) in the United States, and the European Medicines Agency (EMA), to obtain approval for production and sales. However, if a quality problem occurs, such as a quality defect or a non-conforming lot, possible risks are as follows.
- Decline in product reputation
- Quality defect due to a discrepancy between approval documents and actual manufacturing conditions, shipment suspension, product recall, or suspension of business due to administrative action
- A product recall due to lack of data integrity or a significant indication in an inspection by a competent agency
- Decline in credibility of the company
≪ Major responses and initiatives≫
- Establishment and promotion of the SHIONOGI Group Quality Policy
- Holding educational events, etc. to increase employees’understanding of the importance of quality
- Promoting activities to foster a “quality culture”
- Management and supervision activities through audits of manufacturing facilities, etc.
- Strengthening the Bad News Fast/First system through collaborative meetings with relevant internal departments
2.Global supply chain management
≪ Risk Summary ≫
If any problem occurs in the supply chain due to a natural disaster, such as a large earthquake, storm, or flood, the outbreak of a pandemic, a geopolitical influence, such as the economic conflict between the U.S. and China or a Taiwan emergency, or a sustainability-related factor, such as relating to human rights or the environment, the possible risks are as follows.
- Suspension of plant operation
- Difficulty in procuring raw materials and products
- Disruption of wholesale distribution network and stagnation of information
- Significant impact on stable supply of pharmaceuticals
- Adverse impact on society and public health
≪ Major responses and initiatives ≫
- Inventory management based on unique standards for inventory levels to be held
- Establishment of a system for the manufacture of active ingredients contained in some products in Japan
- Diversifying raw material procurement sources to ensure a stable supply of products (selecting second vendors for raw materials with high geopolitical risk)
- Establishing and periodically reviewing BCP items to be supplied on a priority basis
- Conducting due diligence and audits on suppliers and requesting improvements
- Obtaining consent from suppliers to the SHIONOGI Group Business Partner Code of Conduct
- Consideration for business partners in accordance with the “multi-stakeholder policy.”
- Smooth cooperation with wholesalers, and discussion and planning of disaster countermeasures with them
- Ensuring transparency of wholesale and market inventory
- Review of the crisis management system and crisis management regulations
3.IT security and information management
≪ Risk Summary ≫
SHIONOGI holds a large amount of confidential information, including personal information, and uses various IT systems, including those of outsourced contractors. If its IT security is threatened by a willful or negligent act of an employee or contractor, or a cyberattack by a malicious third party, the possible risks are as follows.
- Difficulty in continuing business due to suspension of an important system
- Leakage of confidential information, including personal information
- Incurring of legal damages, such as claims for compensation for damage, and costs related to post-incident measures
- Decline in business performance and reputation
≪ Major responses and initiatives ≫
- Conducting education to ensure that all employees of the Group are aware of the importance of information management and personal information and the need to comply with laws and regulations regarding the protection of personal information, as well as education on the SHIONOGI Group Global Privacy Policy
- Promoting a project to establish an IT-BCP system to be prepared for a cyberattack, a large-scale disaster, or any other crisis
- Improving IT infrastructure, strengthening information security infrastructure, and improving the infrastructure’s operations
- Enhancing the Group-wide network structure based on a global security assessment conducted for the entire Group
4.Adverse drug reaction (ADR)
≪ Risk Summary ≫
Pharmaceuticals are approved and sold after rigorous review by competent authorities around the world. After launch, efforts are made to gather safety information and take necessary measures for ensuring safety and proper use of pharmaceuticals. In addition, the Company, as a HaaS company, is expanding sales of products and services other than pharmaceuticals. Since there are some areas of products and services for which global review standards have not yet been established globally, it is important to ensure the safety of the products and services themselves through individual discussions with competent authorities. It is also important that products and services, including pharmaceuticals, are used appropriately. We therefore provide information to our customers and work to raise their awareness about the relevant diseases. In the event of an unforeseen adverse drug reaction (ADR) or problem in pharmaceuticals or product services, failure or delay in reporting ADR, delay in responding to problems, or an error in the information provided by the Company, the following risks are expected.
- Suspension of sales or product recall
- Lawsuits filed for compensation for health damage
- Impact on business performance and reputation
≪ Major responses and initiatives ≫
- Establishing and strengthening systems to properly gather, analyze, evaluate, and report on safety-related information, such as side effects and other problems.
- Conducting education for all employees that leads to minimizing the spread and harm of side effects, problems, etc.
- Implementing product safety training for management
- Coverage by insurance for indemnity of medical damage from side effects, etc. of pharmaceuticals
- Implementing expert supervision and internal review of information provided and educational content, as well as regular updates to content
5.Compliance
≪ Risk Summary ≫
SHIONOGI recognizes that compliance not only refers to the observance of laws, rules, and regulations but also includes compliance with social norms and ethical behavior as a company and a member of society. Under this awareness, violations of laws and regulations, deviations from social norms, and unethical behavior/actions in the course of business activities are considered as material risks. If any of such risk emerges, the possible impacts are as follows.
- Decline in reputation
- Loss of trust from stakeholders
- Worsened business results and financial condition
≪ Major responses and initiatives ≫
- Establishment and promotion of the SHIONOGI Group Code of Conduct
- Enhancing compliance awareness of all Group employees through Global Compliance & Quality Week
- Operation of the compliance promotion system according to the structure of organizations
- Meetings of the Compliance Committee chaired by the Representative Director, President and CEO (four times a year)
- Reporting to the Board of Directors on the status of the Compliance Committee activities (twice a year)
- Conducting compliance awareness surveys among all employees, and providing feedback on analysis results for each organization
- Providing training to all employees globally on the checklist of five items for employees to stop and reassess their actions when they are unsure of what to do
6.Environment and safety
≪ Risk Summary ≫
In the course of business activities, such as pharmaceutical research, development, and manufacturing, events that affect the environment and/or ecosystems may occur. If damage caused by such events emerges, the possible risks are as follows.
- Suspension of operation of facilities or equipment, or incurring of countermeasures or recovery/repair costs
- Lawsuits filed for compensation, or payment of compensation costs
- Decline in business performance and reputation
≪ Major responses and initiatives ≫
- Establishment of the EHS Policy and the EHS Code of Conduct to enhance governance
- Establishment of a Group-wide environment, health and safety (EHS) management system
- Promotion of Medium-Term Action Plan regarding environmental materiality and EHS
- Strengthening the operation of ISO 14001 and ISO 45001 and the EHS management system in accordance with these standards at each operating site
- Ensuring compliance with relevant laws and regulations, and formulating stricter voluntary management standards and targets
7.Partnerships with other companies
≪ Risk Summary ≫
The purpose of collaboration with business partners is to enable mutual provision of management resources and internal information and to strengthen business by utilizing the strengths of both parties. However, it is associated with the risks below.
- Use of SHIONOGI’s technologies and know-how by partners for purposes other than the purpose of the business alliance
- Lawsuits due to unintended, unauthorized use of technologies of other companies or infringement of intellectual property by SHIONOGI
- Leakage of confidential information by SHIONOGI
- Decline in the brand image, reputation, or trust from investors due to leakage of confidential information by other companies
≪ Major responses and initiatives ≫
- Enhancing communication with partners to eliminate misunderstanding and maintain or improve relationships of trust
- Entering into a non-disclosure agreement incorporating potential risks
- Entering into a contract that clarifies matters related to handling of intellectual property rights and compensation for damage
- Avoiding litigation risks by conducting periodic inspection of intellectual property to identify problems and infringement risks
- Establishment of an information management system by properly encrypting data, strengthening access control, preventing unauthorized access from outside using a firewall, and developing a security monitoring system
- Minimizing information to be shared with partners and formulating rules for information sharing
- Establishment of a system to monitor the status of use of shared information and access logs
- Regular audits and evaluation of the information management system of partner companies
- Conducting due diligence from multiple perspectives on the reliability, financial condition, legal issues, etc. of partner companies
- Conducting regular audits and evaluation of partnerships for the purpose of early detection of problems and points requiring improvement
8.Intellectual property
≪ Risk Summary ≫
SHIONOGI’s products generate profits under the protection of intellectual property rights (e.g., patents). However, due to factors such as an increase in Group companies and changes and expansion of business areas, there is a risk that various intellectual properties may not be sufficiently protected or that third parties’intellectual property rights may be infringed. If the intellectual property rights SHIONOGI owns are infringed by a third party, or SHIONOGI’s products infringe the intellectual property rights of a third party, the possible risks are as follows.
- Deterioration of business results and financial condition due to loss of expected revenue
- Disputes or lawsuits for the protection of intellectual property rights
- Payment of damages
- Injunction against manufacture and sale of the product
- Decline in the corporate brand or reputation
≪ Major responses and initiatives ≫
- Proper acquisition of intellectual property rights and establishment of a management system, and continuous surveillance on infringement of rights by third parties
- Conducting infringement prevention surveys in business activities
- Putting in place a system to prevent infringement by carrying out IP due diligence in in-licensing and out-licensing activities, etc.
- Conducting education for employees through e-Learning, etc. on a regular basis
9.Systems and governments
≪ Risk Summary ≫
The Pharmaceuticals Business is subject to a range of regulations due to various government policies in each country and region. In addition to tighter health insurance finances, the U.S. Inflation Reduction Act (IRA) and other laws may further increase the pressure to curb drug cost increases, especially in developed countries. We also need to pay close attention to the impact of the changes in government in the U.S. and other countries around the world on their policies and international relations. In Japan, government policy trends, such as the health insurance system reforms in anticipation of an increase in healthcare expenses due to the further aging of the population and the annual NHI price revisions, may affect SHIONOGI’s performance. In addition, changes in regulations in Japan and overseas related to the development and manufacture of pharmaceuticals may result in additional costs or the need for new measures. If any of such events emerges, the possible risks are as follows.
- Decline in the predictability of the prescription drug business
- Determination of drug prices deviating from the created innovation value
- Delay in research and development, or supply instability of drugs, vaccines, etc.
- Decrease in sales and profits of drugs, vaccines, etc.
≪ Major responses and initiatives ≫
- Creating innovative pharmaceuticals and healthcare services and providing them at prices acceptable to society
- Building evidence that demonstrates the value of its innovations
- Promoting efforts to appeal to the value of innovations through the activities of industry associations
- Obtaining the latest information on the NHI drug price system and various regulations on the research and development, manufacture, and sale of pharmaceuticals, etc., and promptly responding to them
Beyond the above significant risks, there are various other risks that may affect SHIONOGI’s business performance and financial condition, such as those related to litigation, pandemics and natural disasters, and financial markets and foreign exchange trends. Those listed here do not represent all the risks for SHIONOGI.
Crisis management system
Business continuity planning (BCP)
With respect to the supply of pharmaceutical products, in fiscal 2018 we established Shionogi Pharma Co., Ltd. to take charge of manufacturing operations. At that time we conducted a BCP review, resulting in a rebuild of both our business continuity management system. In addition to the above, we regularly conduct training at both the management and front-line levels to ensure the ongoing viability of operations.
Overview of business continuity management system
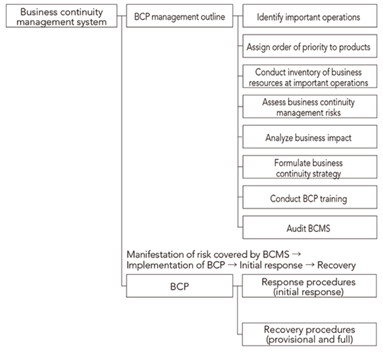
Establishing a stronger system in light of COVID-19
Our goal is to be a drug discovery-based pharmaceutical company for which infectious diseases are a core therapeutic area, and which, while confronting infectious diseases, is resilient to all threats, including pandemics. In response to COVID-19, we are taking measures to tackle various issues that became clear in the course of continuing the business, and we have established robust systems for business continuity ready for a second and third wave of the spread of the virus. In doing so, we are determined to fulfill our responsibilities to our stakeholders
while balancing the sustainability of social and economic activities.
Specifically, by taking a detailed look back at our response, we will be reviewing our BCPs so that we can flexibly respond to the re-expansion of COVID-19 or the occurrence of a new pandemic. Furthermore, we are building more-effective BCP systems through repeated BCP-based simulation training. In parallel, we are continuing to build a more robust IT system.
