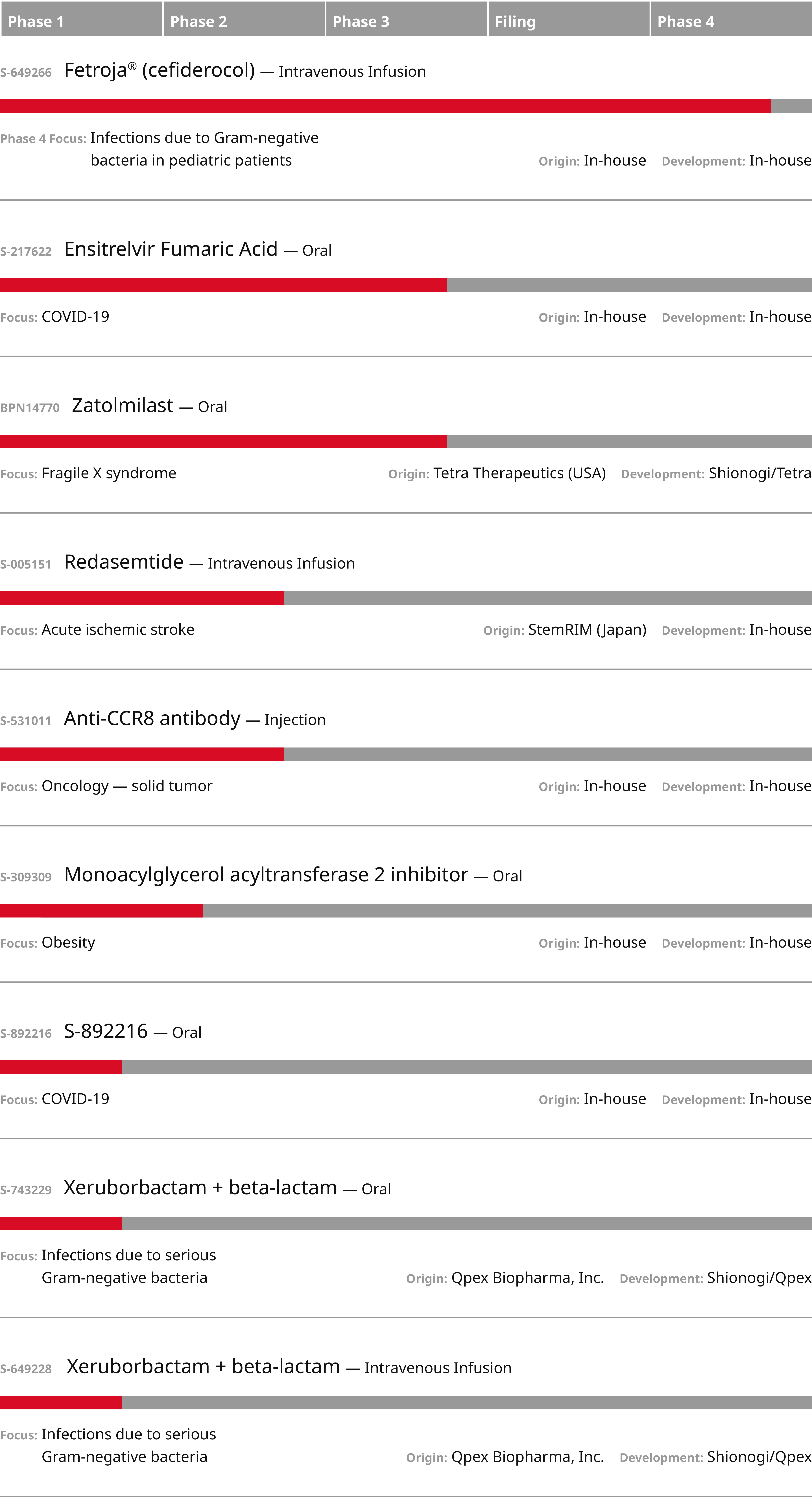
Pipeline
We are committed to identifying unmet needs and harnessing the full potential of science to treat challenging human diseases. Our discoveries have led to the development of new treatments that have improved patients’ lives worldwide.
Shionogi has a long history of small molecule drug discovery. Our current pipeline includes projects across several therapeutic areas, including infectious disease. We are advancing clinical programs for COVID-19, Fragile X syndrome, acute ischemic stroke, metabolic disorders and oncology.
Learn more about the investigational compounds currently in our U.S. pipeline below.
Featured Assets
Ensitrelvir Fumaric Acid (S-217622)
- Stage: Phase 3
- Focus: COVID-19
- Compound: Small Molecule
- MOA/Administration: 3CL protease inhibitor (oral)
- Origin/Development: Shionogi
Addressing Unmet Medical Needs:
There remains an urgent need for a range of treatment options to help address COVID-19 worldwide. Ensitrelvir is an investigational 3CL protease inhibitor, administered orally once daily for five days, being evaluated as an antiviral treatment for COVID-19. Ensitrelvir works by blocking the 3CL protease (an enzyme essential for SARS-CoV-2 to replicate within the human body), which may help combat the illness caused by the virus by inhibiting its replication, potentially preventing progression to more severe symptoms and stages of COVID-19.
Cefiderocol (S-649266)
- Stage: Phase 4
- Focus: Potential additional patient population, infections due to Gram-negative bacteria in pediatric patients
- Compound: Small Molecule
- MOA/Administration: Cephalosporin antibacterial with activity against Gram-negative aerobic bacteria (intravenous infusion)
- Origin/Development: Shionogi
Addressing Unmet Medical Needs:
Shionogi is evaluating cefiderocol in pediatric patients, birth to 17 years of age. Multidrug-resistant and carbapenem-resistant Gram-negative infections affect children globally.
Zatolmilast (BPN14770)
- Stage: Phase 3
- Focus: Fragile X syndrome (FXS)
- Compound: Small molecule
- MOA/Administration: Oral phosphodiesterase-4D (PDE4D) allosteric inhibitor
- Origin: Tetra
- Development: Shionogi/Tetra
Addressing Unmet Medical Needs:
FXS is a genetic disorder that causes intellectual and developmental disabilities and is a common cause of autism. Zatolmilast is an investigational drug that is believed to work by increasing a signaling compound called cAMP, which when increased may promote the maturation of connections between neurons that are impaired in patients with FXS. Zatolmilast is in Phase 3 clinical trials and has received orphan drug designation from the U.S. FDA.
Redasemtide (S-005151)
- Stage: Phase 2b
- Focus: Acute ischemic stroke; additional indications under consideration
- Compound: Biologic
- MOA/Administration: Regeneration-inducing medicine (intravenous infusion)
- Origin: StemRIM
- Development: Shionogi
Addressing Unmet Medical Needs:
Redasemtide is an investigational medicine that is believed to work by inducing functional regeneration of tissues and organs damaged due to injury or illness by mobilizing bone marrow mesenchymal stem cells. Shionogi is advancing the development of this compound in acute ischemic stroke globally. Redasemtide is also being studied in Japan for epidermolysis bullosa.
U.S. Pipeline
These compounds and their uses are investigational and have not all been approved by the U.S. Food and Drug Administration. This information is presented only for purposes of providing a general overview and should not be construed as a recommendation for use of any product for unapproved uses.