Research Topics

At SHIONOGI, we seek to continue creating new drugs that balance innovation with healthcare affordability, by investing appropriate sums in R&D and maintaining small molecule drug discovery as a key strength while also diversifying into nonstandard peptide drug discovery and deepening collaboration with all manner of partners including the IT industry, in order to expand into new modalities and acquire entirely new technologies.
Protect people from the threat of infectious diseases
Acute infectious diseases, incl. pandemic response
COVID-19
COVID-19 spread around the world immediately after its outbreak, having a major impact not only on human health, but on political, economy and lifestyles. Even now, four years after the outbreak, the aftermath continues, and its scars remain in society.
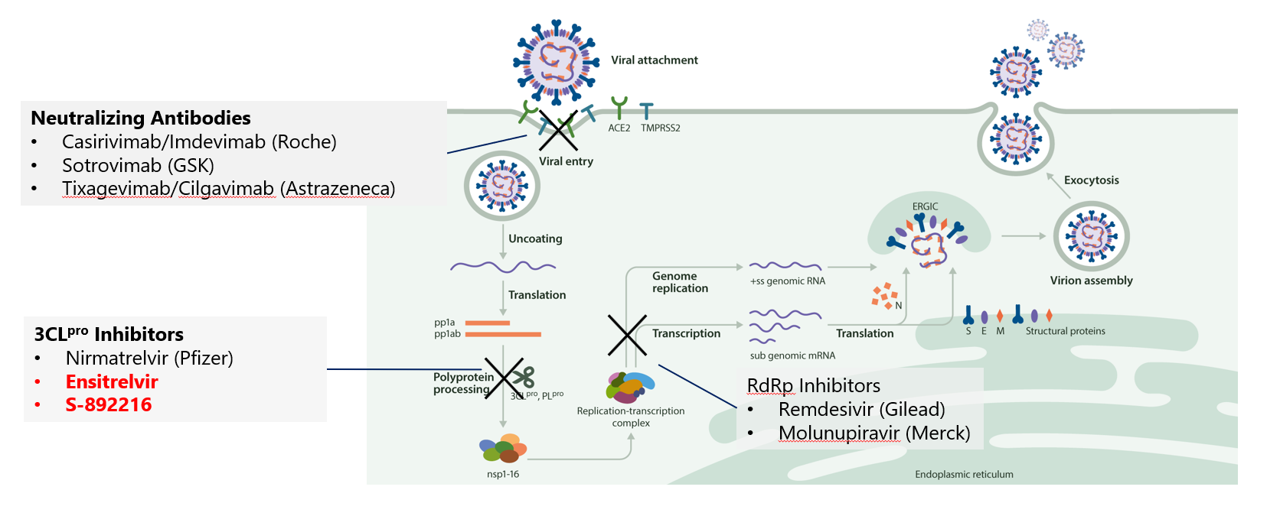
While pharmaceutical companies around the world are striving to overcome the unprecedented battle against this virus that has destroyed society, SHIONOGI has made maximum use of its in-house antiviral drug research know-how, resources, and external network, has broken “Usual” of its own research process, leading to the creation of a unique new protease inhibitor, Ensitrelvir. We are working every day to deliver Ensitrelvir as quickly as possible, first from Japan, and then to patients around the world.
However, the COVID-19 epidemic has not yet been controlled, and many patients are suffering from the aftereffects. As the company SHIONOGI for infectious diseases, we will continue to engage in original innovative research and development, and fight to provide solutions on the front lines of infectious disease prevention and treatment.
RS Virus
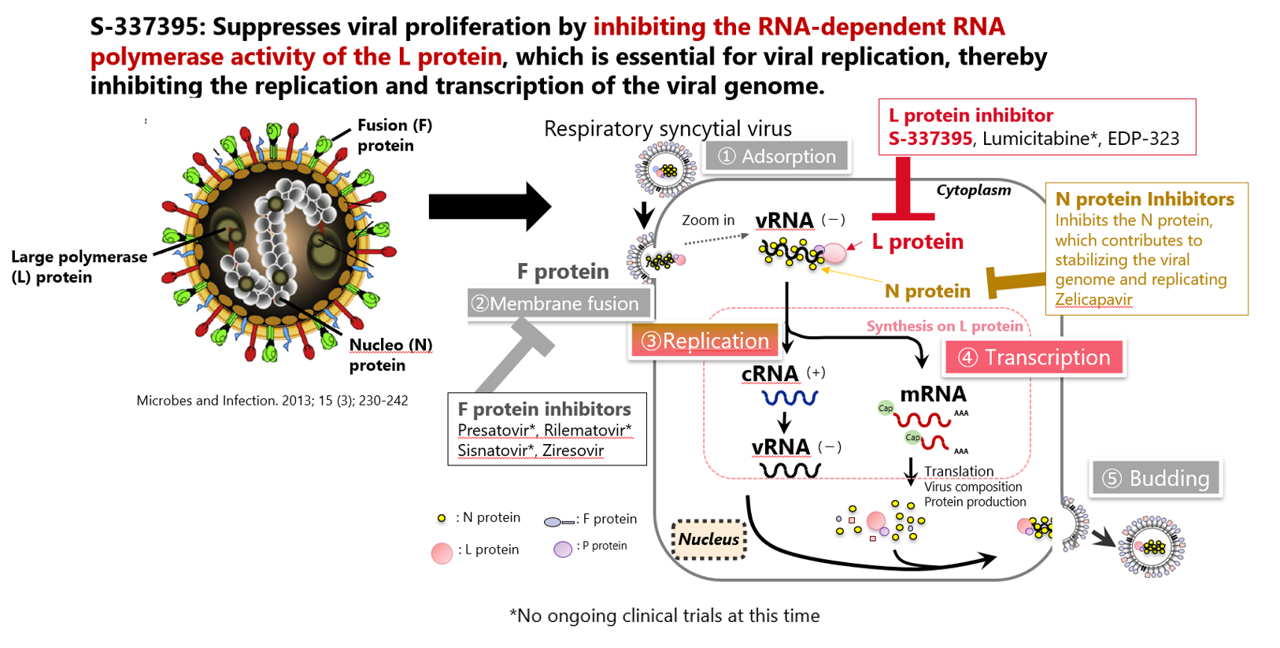
Respiratory syncytial virus (RS virus) is a pathogen that causes severe symptoms such as bronchiolitis and pneumonia, especially in infants. If infected within a few weeks to a few months after birth, the disease may become severe and even lead to death. In addition, low birth weight infants, elderly people, individuals with underlying cardiopulmonary diseases, and those in an immunosuppressed state are more likely to develop lower respiratory tract infections and pneumonia. This increases the risk of the conditions becoming severe, necessitating extra caution. In particular, in the elderly, due to advancements in diagnostic technology, it has become increasingly clear that these symptoms can be attributed to an RS virus infection. Thus, despite the large number of patients threatened by the RS virus, there are no therapeutic drugs available. This highlights the high level of unmet medical needs associated with this disease.
SHIONOGI is engaged in drug discovery research every day with the aim of relieving patients from infectious diseases. For RS virus with high unmet medical needs, we target an enzyme called RNA-dependent RNA polymerase in the L protein, and combine our know-how in antiviral drug research with UBE's ability to synthesize small molecules, led to the discovery of a new compound, S-337395. Clinical trials are currently underway.
There are still many infectious diseases with no cures. SHIONOGI continues to deliver therapeutic drugs through original research and development as soon as possible, in order to relieve people who are suffering from infectious diseases.
Antimicrobial resistance (AMR) infections
Cefiderocol
Infectious diseases caused by AMR bacteria is called a "silent pandemic" that creeps up without people being aware of it, and is a threat to humankind. In particular, the emergence and spread of carbapenem-resistant Gram-negative bacteria, such as carbapenem-resistant Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter spp., has become a global problem, and the World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) have issued warnings that new antibacterial drugs are urgently needed for drug-resistant bacteria.
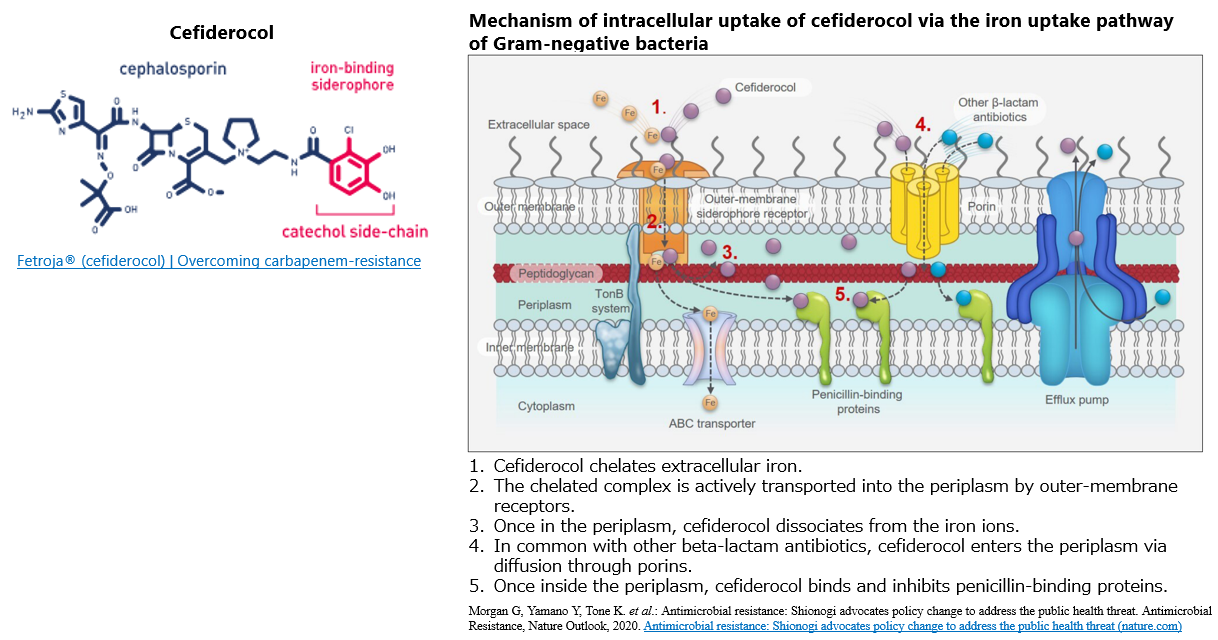
Cefiderocol is an injectable siderophore cephalosporin with antimicrobial activity against Gram-negative bacteria, including carbapenem-resistant strains, approved in the US and Europe in 2019-2020. In Japan, it was approved in 2023 as an antibiotic for the treatment of various infectious diseases targeting bacterial strains resistant to carbapenem antibiotics.
It has the advantage of being able to efficiently enter the bacterial cells via the iron uptake pathway, which is essential for bacterial growth. Such a unique mechanism is called a "Trojan horse".
In addition, cefiderocol is highly stable against all four Ambler classes of β-lactamases that hydrolyze β-lactam antimicrobials. As a result, it exhibits strong antibacterial activity against Gram-negative bacteria that produce various carbapenemases, making it possible to overcome carbapenem resistance.
Shionogi will continue to work on new drug discovery to solve the problems of patients and society, aiming to be free from the threat of AMR infectious diseases, which is expanding on a global level. In order to solve the problems, in 2023, we acquired the US-based Qpex Biopharma, Inc. We will continue to work on new drug discovery by utilizing Qpex Biopharma, Inc.'s new developments for AMR, its capabilities in antibacterial research and development, and its external network in the US.
Infectious diseases that require long-term treatment
HIV
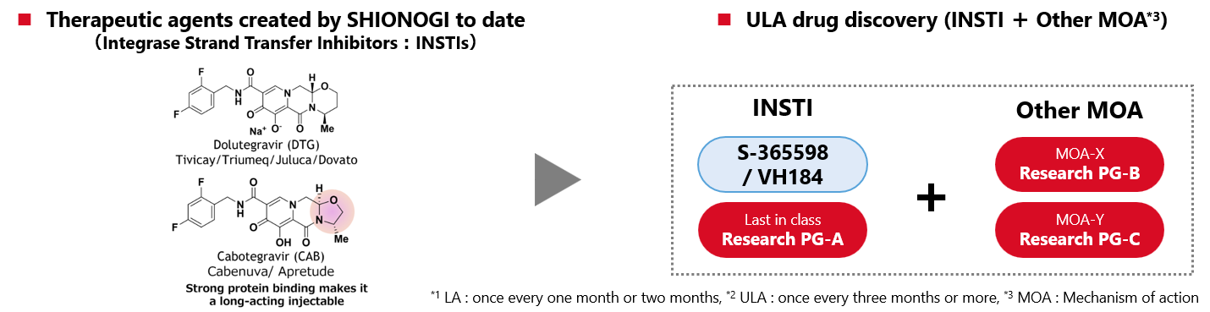
In recent years, there has been a growing demand for sustained-release long-acting (LA*¹) formulations to address unmet needs that cannot be fulfilled by oral medications. Cabenuva is approved for once-monthly and bimonthly dosing in Europe and the US, and is also approved for once-monthly dosing in Canada. This enables the treatment of HIV with just six administrations per year.
In addition, for cabotegravir monotherapy, Apretude, the world's first long-acting injectable drug for the prevention of HIV infection, has been approved for the prevention of HIV infection.
At SHIONOGI's research institute, Our primary focus lies in the development of ultra-long-acting (ULA*²) formulations that provide sustained effects over an extended period and are more convenient to administer so that HIV patients can choose new treatment methods that match their lifestyles and receive appropriate treatment and prevention. To date, we have created a new integrase inhibitor, S-365598 (VH184), which has been licensed to ViiV, and is currently working on multiple research programs (PG).
Vaccine
SHIONOGI will start a full-scale vaccine business to realize total care for infectious diseases. So far, with UMN Pharma as a wholly owned subsidiary, we have promoted the creation of vaccines using the BEVS1) production technology for recombinant protein vaccines using viral antigen proteins with established rhabdo-free2) insect cell culture technology. SHIONOGI's COVID-19 vaccine is a recombinant protein vaccine that combines an adjuvant3) with an antigen produced by BEVS technology. Starting from the launch of our first recombinant protein vaccine "COVGOZE" in June 2024, we will provide the products that meet unmet needs to our society, while accumulating a track record as a vaccine manufacturer.
In addition to the COVID-19 vaccine, we are also working on the creation of vaccines for respiratory infections such as influenza. The challenge in creating vaccines against novel coronaviruses and influenza viruses is that mutations in the causative viruses evade immunity, weakening the effectiveness of existing vaccines. SHIONOGI is working on research and development of a universal vaccine4) to cut off this negative chain and to combat the next pandemic that may occur. For the new coronavirus vaccine, with the support of SCARDA5), we will collaborate with KOTAI Biotechnologies, Inc. which has strengths in antigen design and analysis of immune status, and the National Institute of Infectious Diseases, a leading Japanese institution in infectious disease research. We are working on the creation of a universal vaccine that covers all sarvecoviruses, including the new coronavirus6).
In the prevention of respiratory infections caused by SARS-CoV-2 and influenza viruses, it is considered very important to induce mucosal immunity in the mucosa of the nose and throat to prevent viral infection itself. Aiming to create nasal vaccines that can effectively induce mucosal immunity, we are conducting research in collaboration with the Department of Human Mucosal Vaccinology7) established at Chiba University Hospital.
Adjuvants are substances given with vaccines that are used to make the vaccine more effective. To date, SHIONOGI has created a squalene-based oil-in-water emulsion adjuvant A-910823, and a novel Toll-like receptor 9 agonist adjuvant S-540956, which is an improved B-type CpG ODN2006. We will further develop the technology platform that we have cultivated through these activities, and promote our research aimed at selecting the optimal vaccine adjuvant for enhancing immune response.
1) BEVS: Baculovirus Expression Vector System
2) rhabdo-free: not contaminated with rhabdovirus3) adjuvant: a substance that enhances immunogenicity
3) adjuvant: a substance that enhances immunogenicity
4) Universal vaccine: Vaccine with broad-spectrum that crosses all viruses
5) SCARDA: Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response
6) Adoption of Universal Antigen Vaccine Research and Development for Vaccine and New Modality Research and Development Project by AMED (Press release, June 30, 2022) | Acceptance | Shionogi Pharmaceutical (Shionogi) (shionogi.com)
7) Shionogi Announces the Conclusion of a Contract for the Establishment of a Research Department for Mucosal Vaccines with Chiba University Hospital. (Press Release, February 10, 2022) |Shionogi Pharmaceutical (Shionogi) (shionogi.com)


Contribute to a healthy and prosperous life
Dementia
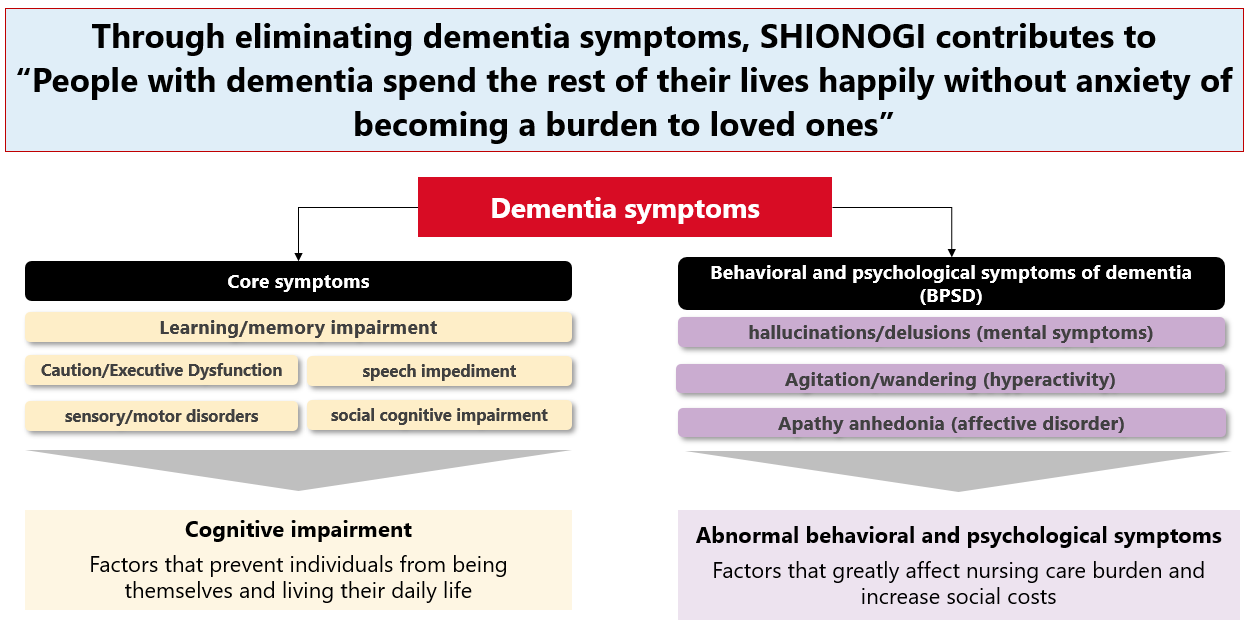
The number of people with dementia currently exceeds approximately 55 million people worldwide*1, and is expected to increase to 76 million by 2030 and 135 million by 2050*2. Recent approvals of an amyloid antibodies, disease modifying agent, for Alzheimer’s dementia give patients and their families a long-awaited hope. Indeed, the drugs slowed the progression but failed to halt it. In addition, they cannot be applied to the other types of dementia. The social cost of dementia in Japan is estimated to be 14.5 trillion yen*3, where Approximately 90% are related to be nursing care. One reason for the increase in the cost of care is symptoms related to dementia. They include not only cognitive impairment but also behavioral and psychological symptoms of dementia (BPSD), such as hallucination, delusion, agitation, and wandering. Patients and their families living with dementia are suffering from the symptoms. Through removing the symptoms, SHIONOGI will contribute to “People living with dementia spend the rest of their lives happily without anxiety of becoming a burden to loved ones"
SHIONOGI is conducting research and development to eliminate the symptoms and has various pipelines from the early research to the late stage clinical development. BPN14770, now in the clinic, is a unique candidate that can activate the mechanism involved in memory formation and has the potential to improve learning and memory in dementia as well as psychiatric disorders. In addition, we are promoting research using the prior art drug discovery technologies such as AI to identify the brain mechanisms and drug targets related to BPSD.
※1:https://www.who.int/news-room/fact-sheets/detail/dementia
※2:Global Impact Dementia 2013--2050.(Alzheimer's Disease international)
※3:Research on the economic impact of dementia in Japan (H25-Dementia-General-005)
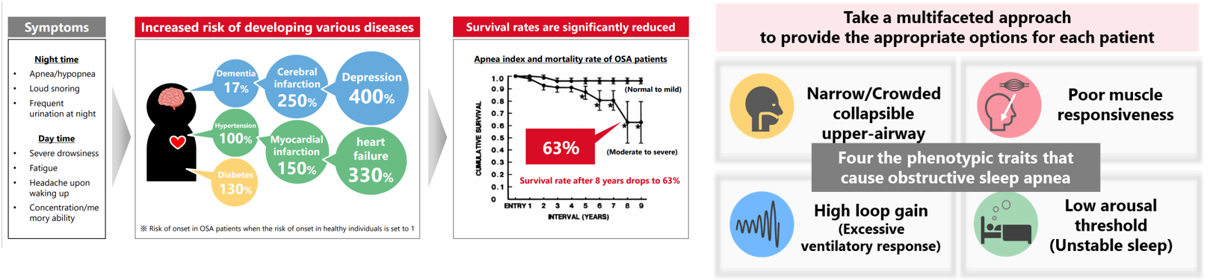
Obstructive Sleep Apnea
Obstructive sleep apnea syndrome (OSAS), a serious sleep disorder, is one of the diseases with high social impact related to decreased labor productivity, and also has risks of complications with chronic diseases such as hypertension, diabetes, cardiovascular diseases, and stroke. If left untreated, it is suggested that lifespan is significantly reduced. In November 2023, SHIONOGI established a joint venture company Shionogi-Apnimed Sleep Science, LLC with Apnimed, Inc. to solve challenges in sleep disorders. By
combining SHIONOGI’s small-molecule drug discovery capabilities with Apnimed’s advanced scientific capabilities, development track record, and global clinical research network, we aim to create promising solutions for OSAS