Improving affordability for patients in need
In developing countries, access to innovative medicines is often hindered due to affordability. Shionogi considers the value and affordability of a medicines carefully and tailors the pricing strategy for each product to the dynamics of the country and healthcare system. Shionogi utilized tools like patient assistant programs, product donations, and patent pools, where appropriate, to improve affordability. In addition, Shionogi intentionally does not file patents for its medications in many low-income and developing countries. This allows other organizations to leverage Shionogi’s research in order to address the needs of patients in these selected markets.

Consideration for inaccessibility due to intellectual property
Intellectual property is an extremely important business asset for pharmaceutical companies. Under Shionogi’s intellectual property strategy, we protect various innovations, such as drug substances, applications, crystalline forms, manufacturing processes, formulations, drug discovery targets and basic research technologies. As part of drug in-licensing and out-licensing activities, we conduct due diligence with respect to intellectual property and take every possible step to prevent Shionogi’s business activities from infringing a third party’s intellectual property. We also carry out brand design activities aimed at building trust in the Shionogi brand and preventing counterfeiting. Shionogi works to protect its intellectual property, employing all legal means necessary if Shionogi’s intellectual property appears to have been infringed.
Shionogi does not consider that the system of intellectual property rights is in itself a barrier to drug access, but we are aware that in certain situations a degree of flexibility is required.
As declared in the Shionogi Group Intellectual Property Policy, until the issue of drug access is resolved, we will refrain from applying for and enforcing patents in developing countries facing economic challenges that are classified as LDCs (Least Developed Countries) or LICs (Low Income Countries).
To Improve Medical Access in LICs/LMICs
In order to achieve universal health coverage (UCH), in which everyone can receive appropriate health services such as prevention, treatment, and rehabilitation at a reasonable cost, the disparity in access to medical care globally must be reduced. However, the factors that impede access to medical care, such as economic, distribution, medical systems, and cultural differences in LICs/LMICs are complex and unique to each country and region, and cannot be solved by SHIONOGI alone. SHIONOGI believes that it is especially useful to form partnerships with international organizations that have excellent solutions to the various issues of access to healthcare in LICs/LMICs.
SHIONOGI have formed partnerships, and strengthened our efforts in the field of infectious diseases, which is considered to be one of the most important issues related to drug access.
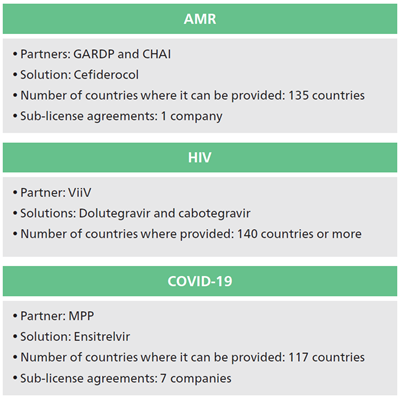
In particular, we are promoting improvements in healthcare access through sub-license agreements with generic manufacturing companies for a total of six products, i.e. cefiderocol, three products related to dolutegravir (Tivicay, Triumeq and Juluca), cabotegravir (Apretude) and ensitrelvir.
Contribution to improving access to antimicrobial resistance (AMR) infectious disease treatments
In June 2022, Shionogi and the Global Antibiotic Research and Development Partnership (GARDP) signed a license and technology transfer agreement for Shionogi's own product, the AMR infectious disease treatment cefiderocol3.
The license territory includes all low-income countries, most lower middle- and upper middle-income countries, and select high-income countries (135 countries total, almost 70% of countries worldwide).

It includes a significant proportion of the world’s population living in areas most affected by AMR. In addition, a collaboration agreement between Shionogi, GARDP and the Clinton Health Access Initiative (CHAI) aim to significantly transform the landscape of access to antibiotics for countries around the world3.
The collaboration agreement includes provisions to work with ministries of health and other experts to strengthen hospital-based stewardship programs that ensure appropriate use. These provisions are especially important to avoid fueling resistance to cefiderocol.
The license and collaboration agreements also offer a dynamic and promising collaborative approach to antibiotic access. By bringing together key actors from the private and non-profit sectors, this project may help overcome barriers so that cefiderocol reaches patients in need.

Contribution to improving access to COVID-19 therapeutic drug
As of February 2022, more than 6.8 million people have died due to the COVID-19 pandemic1. One of the material issues that SHIONOGI prioritizes in particular is “protect people worldwide from the threat of infectious diseases.” As a leading company in infectious diseases, it is SHIONOGI’s mission to combat the threat of COVID-19, which is having a significant impact on the lives, livelihoods, and economies of people worldwide.
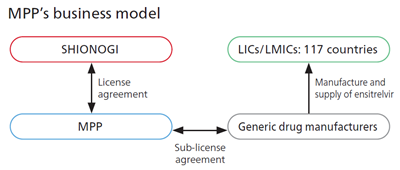
In October 2022, Shionogi signed a voluntary license agreement with the Medicines Patent Pool (MPP)2, a United Nations-backed public health organization, for the COVID-19 treatment ensitrelvir fumaric acid created through joint research between Hokkaido University. This license agreement is intended to be widely available in low- and middle-income countries. This license agreement is the first agreement between MPP and a Japanese company.
Under the terms of the license agreement between Shionogi and MPP, qualified generic manufacturers that are granted sublicences by MPP will be able to manufacture and supply ensitrelvir to 117 countries. Shionogi will waive royalties on sales in all countries covered by the agreement while COVID-19 remains classified as a Public Health Emergency of International Concern by the World Health Organization.
Furthermore, based on the above license agreement, we signed sub-license agreements with seven generic drug manufacturers.3 Through this partnership, we will be able to deliver new treatment options created by SHIONOGI to people living in LICs/LMICs at a fair price.
1. WHO Coronavirus (COVID-19) Dashboard (external site)
Contribution to improving access to anti-HIV agent
Developing countries account for more than 40% of the total global supply volume of the anti-HIV agent Tivicay (dolutegravir), which is licensed to ViiV Healthcare, and its combination drugs Triumeq and Juluca. Registering dolutegravir in the Medicines Patent Pool has allowed generic manufacturers to manufacture dolutegravir, either as a single-ingredient formulation (Tivicay) or combined with other anti-HIV agents (Triumeq and Juluca), and distribute it to more than 140 low-income and lower middle-income countries.
Furthermore, cabotegravir (Apretude), which is licensed to ViiV Healthcare, is registered in the Medicines Patent Pool.
As one of the patent holders of dolutegravir and cabotegravir, Shionogi contributes to this initiative.

