Drug Development Process
Research & Development
The SHIONOGI Group progresses its research and development activities with world-class productivity and with the objective of bringing pharmaceuticals needed by patients rapidly to global markets. The Group is also continuously pursuing access to external innovation through partnership and licensing opportunities with academia and venture companies, focused on future medical needs and novel products and technologies.
Regarding our research efforts, the Shionogi Pharmaceutical Research Center (SPRC), the center of the SHIONOGI Group’s research functions, has continued to serve as a platform through which we have further strengthened the coordination and productivity of our drug discovery efforts. Allowing us to increase the number of development candidates generated and improving the success rate in transitioning from pre-clinical to clinical development. Looking ahead, our goal is to address the needs of a rapidly-aging society (by assisting the extension of the healthy life expectancy, and supporting a return to productive life), focusing on infectious diseases and pain/CNS disorders as core therapeutic areas to create innovative medicines. We will challenge the expansion of new modalities to solve unmet needs that cannot be compensated by small molecules, and we will also strive to provide a broader range of treatment options.
The SHIONOGI Group will continue to work to achieve even more rapid and successful drug development to provide best medicines required for patients globally as quickly as possible using advantage of our experience.
- ※1POC (Proof of Concept): Proof in clinical trials that the compound’s concept (site and mechanism of action), including efficacy and safety, represents a reasonable approach to the treatment of the target disease in humans.
The Drug Development Process:
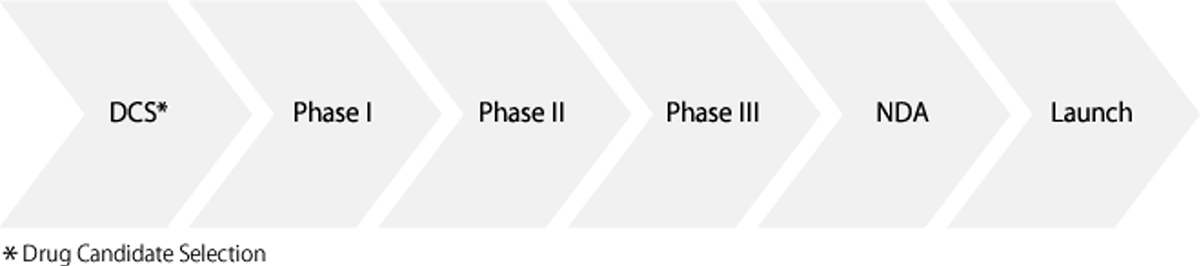
- Phase I Clinical Trials: The initial phase of testing in humans. Confirms the drug’s pharmacokinetics (the rate of absorption in the body and the rate and method of elimination) and safety in healthy adults (certain drugs such as anti-cancer agents, however, are tested in patients).
- Phase II Clinical Trials: Clinical trials to test efficacy and safety by administering the drug to a relatively small number of patients and to determine the effective dose regimen (dosage amount, dosing interval, etc.).
- Phase III Clinical Trials: Clinical trials to test efficacy and safety by administering the drug to a larger number of patients. In these larger clinical trials, the drug’s advantage compared with a placebo or an existing drug is also investigated.


