Partnering
At SHIONOGI, we think sustainable growth hinges not only on new drug creation, but also on consolidating our strengths in areas of strategic focus. In this era of diversifying healthcare needs, there seems to be risk in having both a great deal of infrastructure and not much infrastructure. To maintain large infrastructure, it is necessary also to keep a bulging pipeline. However, this adds to the volume and type of work that in theory should be performed in-house. Through external partnerships, we seek to enhance overall productivity through collaboration in areas where it would be difficult for us to go it alone. By keeping our head office functions comparatively lean while at the same time retaining certain strengths in this ever-changing world, we aim to stay nimble and ensure that our presence is felt.

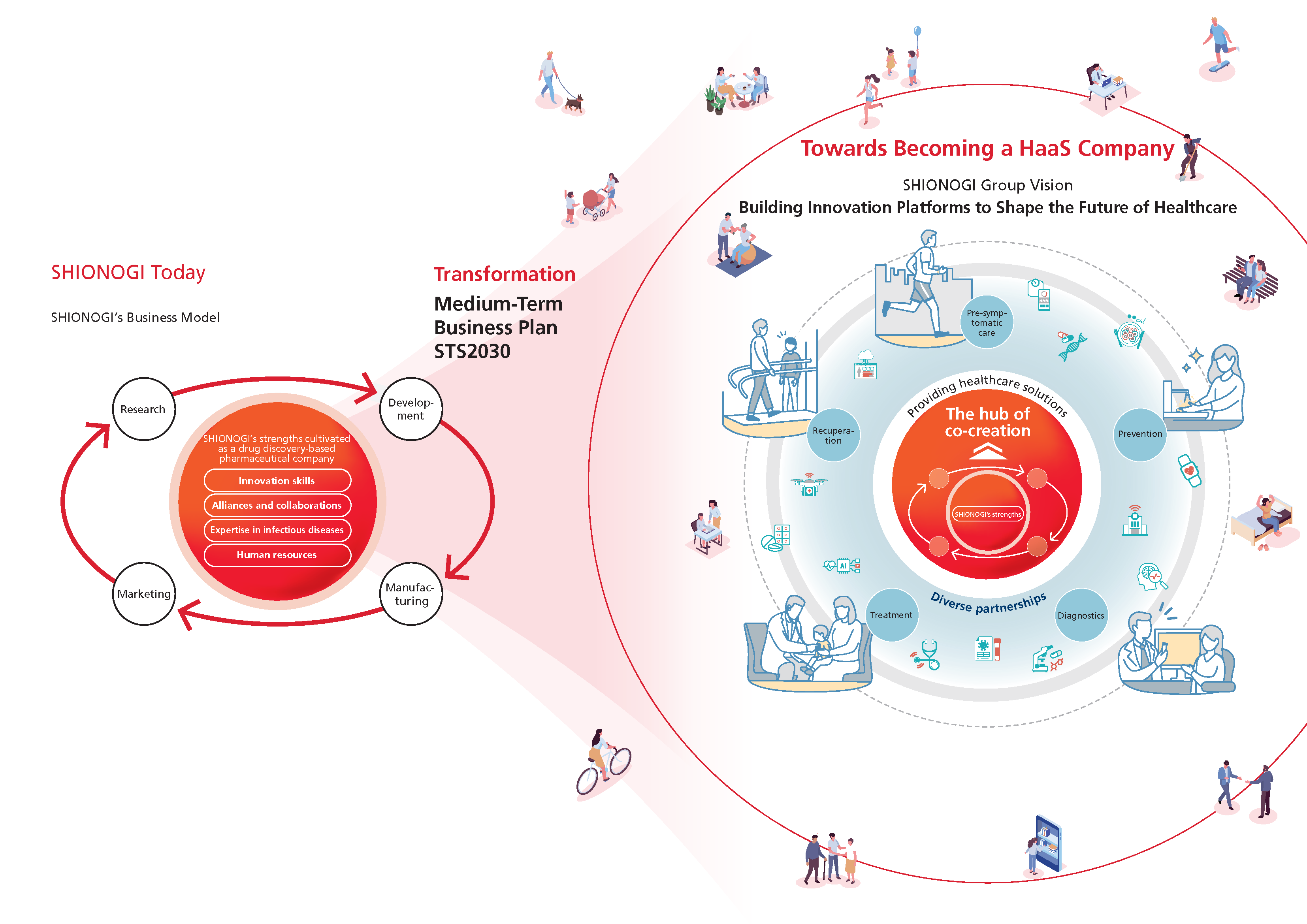
Transformation into a HaaS company
SHIONOGI aims to continue to grow sustainably with society by addressing the needs of patients and the world, and by continuously creating innovations that solve a wide range of healthcare issues. We are committed to transforming ourselves into a HaaS company that provides value beyond the traditional framework of treatment, and are working uncompromisingly every day to deliver cutting-edge healthcare solutions created through innovation to as many people as possible as quickly as possible.