Business Partnership with Nihon University, Gunma University, and Tokyo Medical University for a Rapid Diagnostic Methods for Viruses in the Field of Infectious Diseases, Including Novel Coronavirus
Osaka, Japan, June 22, 2020 – Shionogi & Co., Ltd. (President & CEO: Isao Teshirogi, Ph.D., hereafter: “Shionogi”) announced today that Shionogi has agreed with Nihon University, Gunma University, and Tokyo Medical University on a license agreement regarding a new rapid diagnostic method for viruses including the novel coronavirus (SARS-CoV-2). We will work in collaboration with public institutions, academia including the above three universities, and partner companies to put this diagnostic method into practical use.
With continued social disruption caused by the worldwide spread of SARS-CoV-2, the spread of infection from not only patients with symptoms but also non-progressor and the infected person in the incubation period has become a major issue in the control of infectious disease. At present, as the test methods for diagnosing patients with novel coronavirus infection (COVID-19), PCR (polymerase chain reaction) are used for diagnosing COVID-19 for detecting nucleic acids in viruses from samples collected from the nasal cavity, pharynx and saliva as well as antigen test kits. However, many challenges remain with these testing methods, such as the need for specific detector, the ease and speed of measurement, and the risk of infection of healthcare workers during sample collection. Therefore, there is a need for highly sensitive and inexpensive diagnostic methods that overcome these challenges.
The Joint Research Team, consisting of Nihon University, Gunma University, and Tokyo Medical University, has succeeded in developing a unprecedented innovative viral rapid diagnostic method using signal amplification by ternary initiation complexes method (SATIC method)[1]. The SATIC method is a technology that can measure not only specific genes, but also mutant genes, as well as in-vivo molecules such as proteins and metabolites, using a simple method with high specificity. This rapid diagnostic method using SATIC methods has all of the following features.
・ The presence or absence of SARS-CoV-2 or influenza virus can be easily determined visually without a detector.
・ Virus determination can be made in about 25 minutes after sample collection.
・ There is no non-specific reaction such as false positive reaction, and high sensitivity equivalent to that of PCR.
・ The ability to detect viruses from saliva and sputum as well as nasopharyngeal swabs reduces the invasiveness of the patient and the risk of infection among healthcare workers associated with specimen collection as much as possible. In the case of saliva, specimens can be collected by the patient themselves.
On June 3, 2020, Shionogi launched IgG/IgM Antibody-test Kit for COVID-19 as a research reagent aimed at identifying the number of SARS-CoV-2 infected patients[2]. On the other hand, this diagnostic method allows us to easily know the presence or absence of infection at the time of testing for COVID-19 and influenza virus infections disease, etc. in a short time. As indications for the practical use of this diagnostic method, it is assumed that the presence or absence of infections at medical facilities and quarantines, and in the future, the screening of infections by travellers from abroad will be considered. Since no testing method combines all of the above-mentioned features, the use of this test method enables the diagnosis of SARS-CoV-2 infected individuals, including those without symptoms, quickly and conveniently, and provides a timely understanding of the trends in domestic infected individuals. In addition, it offers the benefits of early diagnosis-based preventive measures against aggravation and early administration of treatment drugs.
Shionogi is committed to “Protect people worldwide from the threat of infectious diseases” as our key focus. We are not limiting ourselves to the research and development of therapeutic medications, but are also focused on the total care of infectious disease, through awareness building, prevention, diagnosis, and treating exacerbations, as well as the infection itself. Shionogi will work closely with government, industry, and academia including the above three universities, and will continue to strive to fulfill the early commercialization of this rapid diagnostic method to fulfill our social responsibility and to contribute to re-establishing the safety and security of society to support early containment of the pandemic.
【About Signal Amplification by Ternary Initiation Complexes method (SATIC method)[1],[3],[4]】
Figure 1: Basic principle of SATIC method
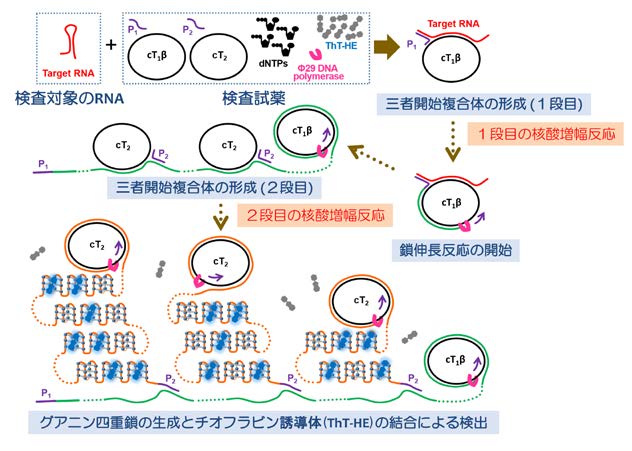
Figure 2: Flow to judgment (procedure)
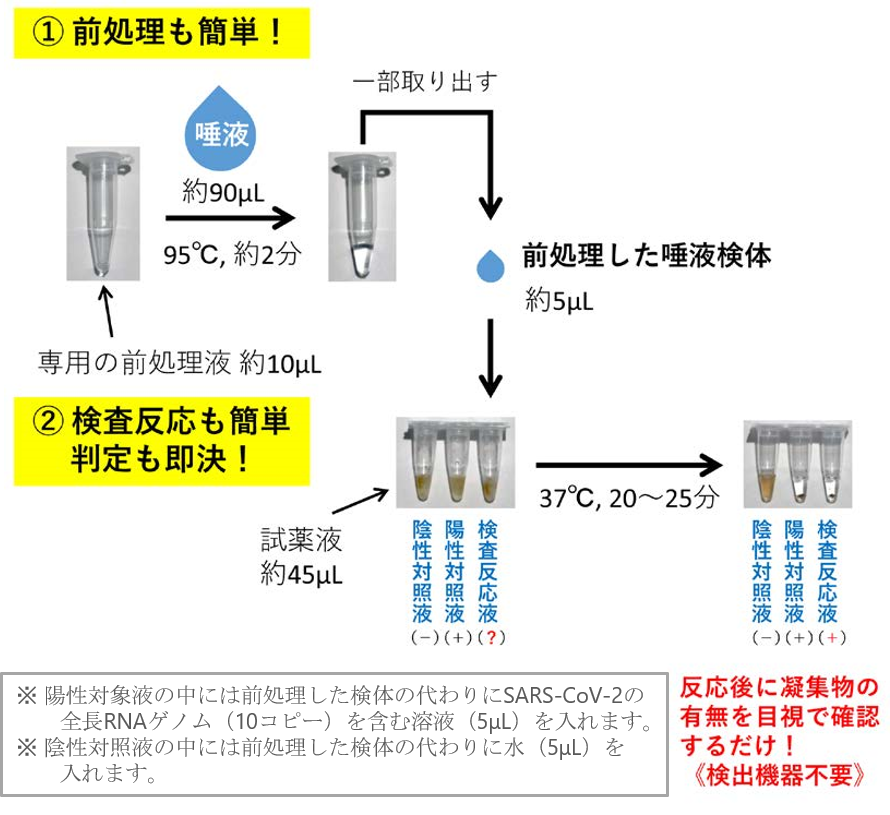
Figure 3: Test results by PCR method
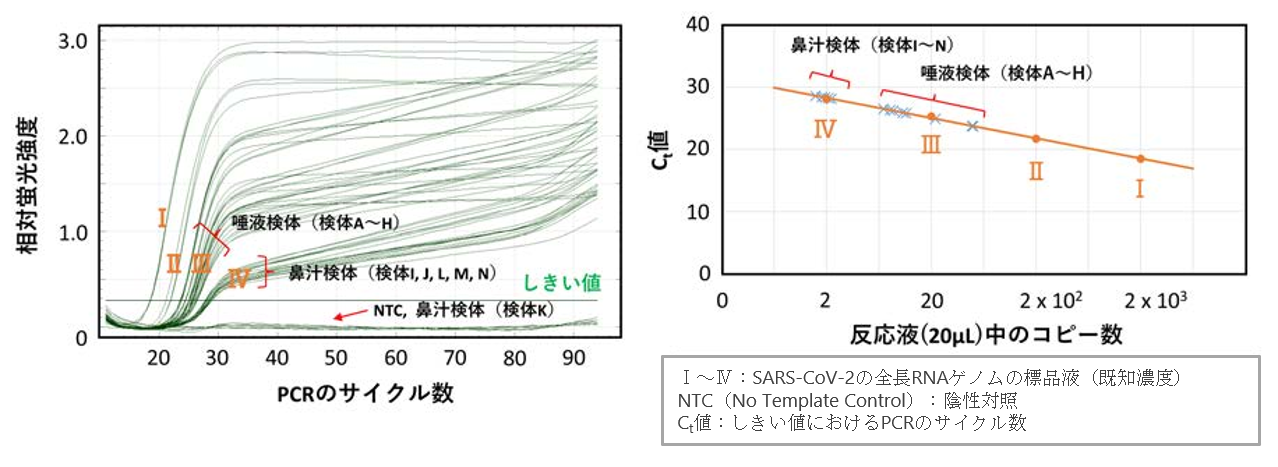
Figure 4: Test results by SATIC method

References
1. Press release on May 14, 2020
Succeeded in developing rapid diagnostic method for COVID-19 using innovative nucleic acid amplification method instead of polymerase chain reaction (PCR)
2. Press release on June 3, 2020
IgG/IgM Antibody-test Kit for COVID-19 launched in Japan
3. H. Fujita et al. Novel one-tube-one-step real-time methodology for rapid transcriptomic biomarker detection: signal amplification by ternary initiation complexes. Anal. Chem. 88 7137-7144 (2016)
4. H. Fujita et al. Specific Light-Up System for Protein and Metabolite Targets Triggered by Initiation Complex Formation. Scientific Reports. 15191 (2017)
