Initiatives
Identification of significant suppliers
The SHIONOGI Group selects suppliers based on the Procurement Policy with due consideration given to geopolitical risks and risks specific to the industries and raw materials that we deal with.
Our domestic group has more than 4,000 suppliers around the world. We designate “suppliers necessary for us to provide the market with our products and services on a stable basis” as significant suppliers and identify such suppliers according to the following steps every yeah.

Supplier risk assessment
The SHIONOGI Group is striving to realize sustainable procurement by establishing a PDCA cycle based on, for example, the formulation of the Business Partner Code of Conduct, the assessment of risks and identification of problems through surveys and audits on ESG elements, and the development of improvement plans through communication with suppliers.
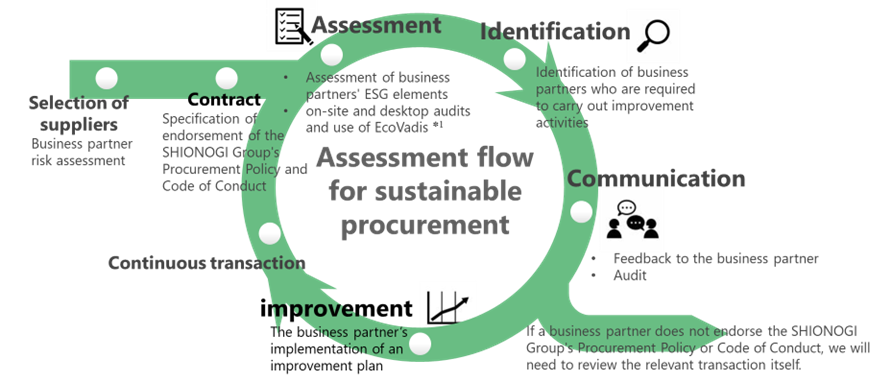
*1 Platform in which scorecards are used to assess a broad range of non-financial management systems including supplier environment, labor & human rights, ethics and sustainable procurement impacts
At SHIONOGI’s manufacturing and research sites, we conduct environmental impact assessment regarding all aspects of their operations from the purchase of raw materials to disposal. Our awareness raising targets not only high-risk in-house operations but also outsourced services, waste disposal operators and other related parties. An emergency communication system is also established between SHIONOGI and its suppliers.
Our Business Partner ESG Management Guidance sets out EHS risk categories and risk management procedures for suppliers and stipulates which conditions they must meet according to their assigned management level. For important investigational new drugs and pharmaceutical raw material suppliers, we carry out surveys using the PSCI’s self-assessment questionnaire (SAQ)—to confirm the supplier’s status in areas including ethics, human rights and labor, environment, health and safety, and their management systems—in conjunction with on-site audits.
Our on-site audits include confirmation of the risks of the relevant suppliers through, for example, assessment of the safety of manufacturing processes and assessment of compliance with the laws and regulations that need to be observed at the operating sites. For issues identified through audits, we agree on corrective action plans with suppliers and have them proceed with improvement activities.
For significant suppliers, we use EcoVadis, a rating platform for fair and objective assessment of corporate social responsibility and sustainable procurement, and assess suppliers in order of priority.
Supplier Management Level and Auditing Items
| № | Category (by handled products) | Management level | Auditing items implemented | ||
|---|---|---|---|---|---|
| Written confirmation | Questionnaire survey | On-site audit | |||
| 1 | Suppliers of API, intermediates or preparations (GMP[※3]–conforming process) for drugs developed since the adoption of PV[※2]–based manufacturing | High | 〇 | 〇 | 〇 |
| 2 | ・Business partners committed to drug substances/ intermediates/drug products/the packaging process (GMP process) for important items※4 ・Business partners with no substitutes for raw materials for important items |
Intermediate | 〇 | 〇 | |
| 3 | Suppliers other than the above (Manufacturers of general-use raw materials, subcontractors in charge of packaging, etc.) |
Low | 〇 | ||
※2 PV: Process validation; confirmation and documentation that the process is valid for permanently manufacturing products of the target quality when operated under a permissible range of conditions set based on the results of industrialization research, the past manufacturing records of similar products, or other such data and in consideration of variables likely to affect the preset product quality (physical properties of raw and other materials, operating conditions, etc.)
※3 GMP: Good Manufacturing Practice: international standards for the manufacturing and quality control of pharmaceutical products; the GMP-conforming manufacturing of pharmaceutical products is required to follow GMP procedures and guidelines concerning operations such as the delivery of raw materials, inspection, manufacturing, packaging, shipment management, storage, and collection and disposal.
※4 Important items: antimicrobial resistance (AMR)-related items and business continuity plan (BCP)-related items
Cooperation with suppliers
The SHIONOGI Group aims to contribute to realizing a sustainable and sound society through business activities in cooperation with suppliers.
For such cooperation, we conduct assessment and selection in accordance with the Procurement Policy. In addition, in order to ensure our commitment in cooperation with suppliers, we exchange necessary documents, such as contracts stipulating their endorsement of our Business Partner Code of Conduct, before proceeding with the transaction. We are working toward receiving the endorsement of all our suppliers.
In addition, the SHIONOGI Group is making efforts to enhance supplier engagement. We are asking significant suppliers to conduct a risk assessment using the EcoVadis platform and implement corrective actions, while offering explanatory sessions and to provide support for addressing ESG challenges. For details on specific initiatives, please see below.
Targets and results
For supplier risk management and cooperation with suppliers, the targets and results for each fiscal year are shown below.
We will continue to work steadily with the cooperation of the entire SHIONOGI Group toward the targets set in light of the entire supply chain, while disclosing our progress.
| Targets and results of initiatives to achieve sustainable procurement | 2023 | 2024 | 2025 | |||
|---|---|---|---|---|---|---|
| Target | Result | Target | Result | Target | ||
| Percentage of suppliers that have endorsed the Business Partner Code of Conduct | New contracts:100% | New contracts:100% | New and existing contracts:100% | New contracts:100% existing contracts:97% |
New and existing contracts:100% | |
| Total number of suppliers that have conducted an ESG risk assessment and corrective actions | 70 | 77 (Cumulative total※5137) |
69 | 95 (Cumulative total139) |
89 | |
| Breakdown (Including different sites of the same company) |
On-site audit (including those outsourced) |
9
|
10 | 9 | 10 | 8 |
| Desktop audit (PSCI SAQ, EcoVadis) |
61 | 68 | 41 | 69 | 70 | |
| Corrective actions | ー | ー | 18 | 16 | 11 | |
| Total number of suppliers that have carried out direct engagement activities related to sustainability challenges | 29 | 28 | 24 | 25 | 15 | |
| Breakdown | Human rights | 14 | 14 | 14 | 14 | 6 |
| Climate change | 15 | 14 | 10 | 11 | 9 | |
※5 Cumulative total: Number of significant suppliers that have conducted an assessment in the relevant fiscal year
For the rationale for setting human rights and climate change targets, please see below.
Training for employees engaged in procurement operations
At SHIONOGI, we are striving to realize sustainable procurement by ensuring that SOPs include the items related to social issues that employees engaged in procurement operations need to understand and follow, such as human rights, labor, and the environment, and by providing a training program so that the employees are fully familiarized with such items.
In FY2021, we provided all the domestic group’s employees, including those engaged in procurement operations, with E-learning training on the Procurement Policy and the Business Partner Code of Conduct. (Participation rate: 84%)
In FY2022, 74 employees whose primary duties involved procurement operations took the SOP training program.
In FY2023, 902 employees who may be involved in procurement operations took the SOP training program.
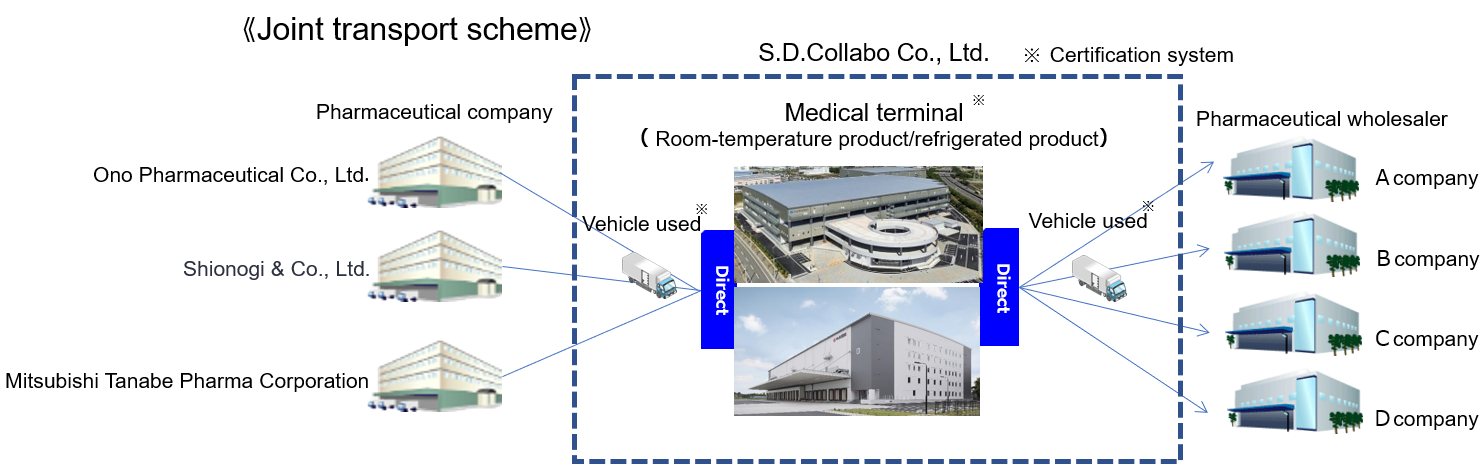
Joint delivery initiatives
In January 2023, we launched a joint delivery in compliance with the Good Distribution Practice (GDP) for pharmaceutical products for the first time in the pharmaceutical industry in cooperation with Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and S.D.Collabo Co., Ltd. Through this joint delivery, we are aiming to achieve the following effects:
- 1GDP compliance: We will improve quality assurance though temperature controlled transportation in all temperature zones.
- 2Address the so-called “2024 problem”: We will mitigate the shortage of drivers and achieve a stable supply by increasing the loading efficiency of transportation and reducing the number of vehicles in operation.
- 3Address environmental problems: We will reduce CO2 emissions by reducing the number of vehicles in operation.
As of October 2025, the number of pharmaceutical companies participating in the joint transportation scheme has expanded to seven.
To maximize the effectiveness of this initiative, we are advancing new efforts.
In terms of GDP (Good Distribution Practice) compliance, we are strengthening our high-quality transportation system in accordance with GDP guidelines. This includes increasing the number of certified vehicles within the scheme, expanding medical terminals, and preparing for cases where certified vehicles are unavailable by utilizing the "VIXELL" temperature-controlled transport box, jointly developed by the Suzuken Group and Panasonic Corporation.
Regarding environmental measures, we are promoting modal shifts by transitioning from conventional truck transport to maritime shipping for mainline routes to Hokkaido, and by exploring the use of domestic rail freight. Furthermore, we are working to reduce environmental impact by promoting paperless shipping labels and other initiatives.